Information for parents and families
The FEED1 trial has now completed recruitment. Please follow this link to the results:
Results
Summary of the study
- Babies who are born early are currently fed small amounts of milk through a tube into their stomach, with additional nutrition through a drip into their veins. We call this "gradual milk feeds" in this study.
- We want to know whether starting babies on "full milk feeds" rather than "gradual milk feeds" will lead to babies going home from hospital earlier.
- To help us understand more, we are comparing two different ways of feeding babies: (1) gradual milk feeds (usual care) and (2) full milk feeds
- We will collect data until your baby is discharged from hospital.
- We will send you a questionnaire to complete once your baby reaches 6 weeks corrected age (6 weeks after their due date).
- We will contact you again (with your permission) when your baby turns 2 years of age to see how your baby is doing.
- If you take part in the study and have given birth to more than one baby, each baby will be fed in the same way, if they are eligible to join the study.
Further information:
Please click the headings below
Why are we conducting this study?
Babies who are born early cannot feed for themselves and are given small amounts of milk through a tube into their stomach. They are also given additional nutrition through a drip into their veins (intravenously or IV). The milk is slowly increased until they are fully milk fed and no longer need any IV nutrients. We call this "gradual milk feeds". We feed premature babies like this because of concerns about a serious bowel disease called Necrotising Enterocolitis (NEC). However, research suggests that for premature babies who aren't too poorly, larger milk feeds can be given without increasing the risk of NEC, and might also reduce the risk of severe infection. In this study we want to find out whether babies born 8-10 weeks early will do better if we feed them fully with milk from the first day. We want to know whether this will help babies go home sooner (reduce the number of days they need to stay in hospital). We will also check if this helps reduce infection risks, affects the risk of NEC, and helps mothers breast feed and be more involved in caring for their baby. The study is taking place in neonatal units across the UK and we want to include 2088 babies in it.
Why have I been asked to take part?
You have been invited to take part in this study as you are either in preterm labour, have a planned delivery 8-10 weeks early, or your baby has been born 8-10 weeks early.
It is up to you whether or not you join the study. We will talk to you about the study and answer any questions you may have. Your babies care will not be affected in any way if you decide you do not want your baby to take part. You are free to withdraw at any time, without giving a reason. This will not affect the standard of care you or your baby receive.
If you have been approached to take part in this study before giving birth, we will ask you to sign a written consent form. If you then deliver 8-10 weeks early, you will participate in the study. If you are about to or have just given birth, you will have already confirmed verbally that you wish to take part in the study, and you will be asked to sign a written consent form at a more practical time. Soon after birth, if the team taking care of your baby thinks your baby is well enough to participate in the study, the decision to use full milk from day 1 or gradual milk feeds will be selected by a computer. The computer will decide randomly and you will have an equal chance of being in either of the two groups. If you have more than one baby from this pregnancy, all your babies will receive the same feeding method. All other feeding decisions will still be made by the doctors and nurses caring for your baby and the rest of your babies care will follow your hospitals usual practice. Whether or not you take part in the study, you will choose the type of milk your baby will be given after talking with the doctors and nurses. Your breast milk is the best milk for your baby. Your midwife, nurses, and doctors will support you to express milk for your baby. If you choose not to use expressed milk or if your milk takes time to come in for the first few days, the doctors and nurses will discuss other options (such as human donor milk or formula milk) that can also be used during the study.
The flowchart below shows what will happen if you decide to take part in the study.
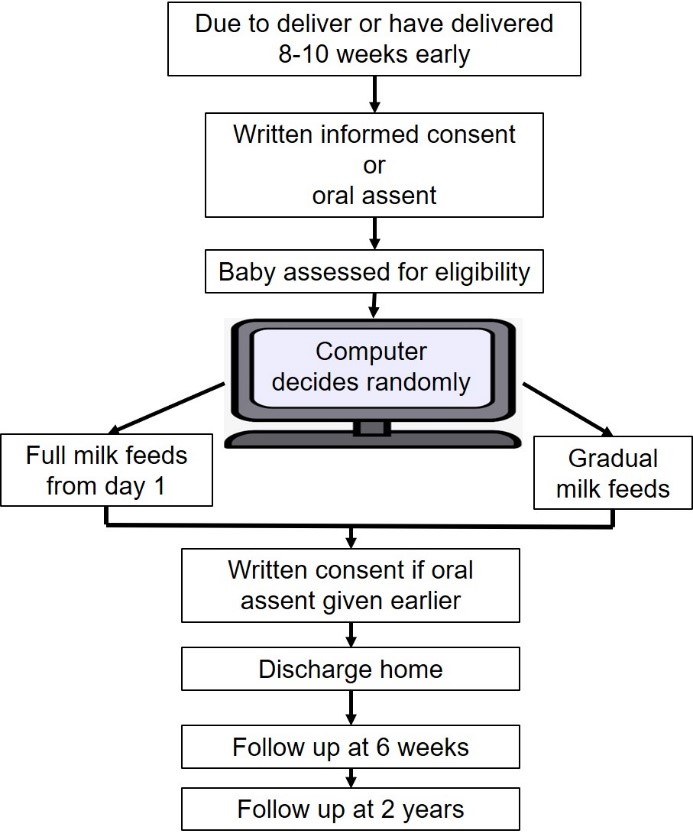
Data about how your babies health, such as how they feed and grow will be collected until your baby goes home. We will send you a questionnaire (either online or in the post) to complete when your baby reaches 6 weeks corrected age.
With your permission, we would like to maintain contact with you as we want to find out how your baby is doing when they reach 2 years of age.
What are the benefits of taking part?
We do not know if taking part in the study will benefit you or your or baby directly, but by doing this study we are hoping to find the best way of feeding preterm babies which may help to guide the care of premature babies in the future.
What are the possible risks of taking part?
If your baby is in the full milk group, they will be given milk from the first day. This amount of milk may be difficult for your baby to tolerate and they may vomit and/or develop bloating. If this happens the doctors and nurses caring for your baby will decide what is best for your baby and may give smaller amounts of milk more frequently or reduce the amount of milk. There may be benefits from full milk from day 1, such as reducing infections and the need for drips associated from IV feeding in the gradual milk group. All babies will be monitored extremely closely throughout the study by the hospital staff. If your baby is unwell or is struggling, the doctor will discuss this with you and do what is best for the baby's care regardless or which group of the study your baby is in.
It is up to you to decide if you want to join the study or not. If you agree to take part, you are free to withdraw at any time, without giving a reason. If you choose not to take part, your care will continue in the normal way.
Who has reviewed the study?
All research in the NHS is reviewed and approved by an independent group of people, called a Research Ethics Committee, to protect your interests. This study has been reviewed and approved by East Midlands Derby Research Ethics Committee.
Will my information be kept confidential?
We will need to use the information from you and your baby/babies medical records for this reserach project. This information will include your initials, yours and your baby's names and NHS numbers and your contact information. People will use this information to do the research or to check yours and your baby's records to make sure that the research is being done properly.
People who do not need to know who you are will not be able to see your name or contact details. A copy of your consent form will be sent to the Nottingham Clinical Trials Unitl (NCTU) but any other information about you which leaves the clinic will be anonymised, meaning your name and address will be removed. Your data will be anonymised with only your initials and Date of Birth and you will be indentified by a code number instead. No one will be able to identify your involvement when the findings are published at the end of the study. The anonymised information collected about you may be used to support other research in the future and may be shared with other researchers.
Your personal contact details will be available to the NCTU so they can contact you during the study and send the questionnaires, they may also contact you to discuss the questionnaire if necessary. Once the study is finished, some of the data will be kept so we can check the results. Your name and email address will be kept after the end of the study so that we can contact you about the findings of the study. If you do not wish to be contacted with the results of the study your name and email address will be disposed of securely at the end of the study. All other data (research data) will be kept securely for 5 years. After this time, your data will be disposed of securely.
We will keep all information about you safe and secure.
You can stop being part of the study at any time, without giving a reason, but we will keep the information about you that we already have.
- If you choose to stop taking part in the study, we would like to continue collecting information about your baby by sending you the follow-up questionnaire. If you do not want this to happen, tell us and we will stop
- We need to manage your records in specific ways for the research to be reliable. This means that we won't be able to let you see or change the data we hold about you.
You can find out more about how we use your information at https://www.uhdb.nhs.uk/research-how-we-use-your-information and www.hra.nhs.uk/information-about-patients/ or by sending an email to feed1@nottingham.ac.uk